Statistical controversies in clinical research: requiem for the 3 + 3 design for phase I trials - Annals of Oncology
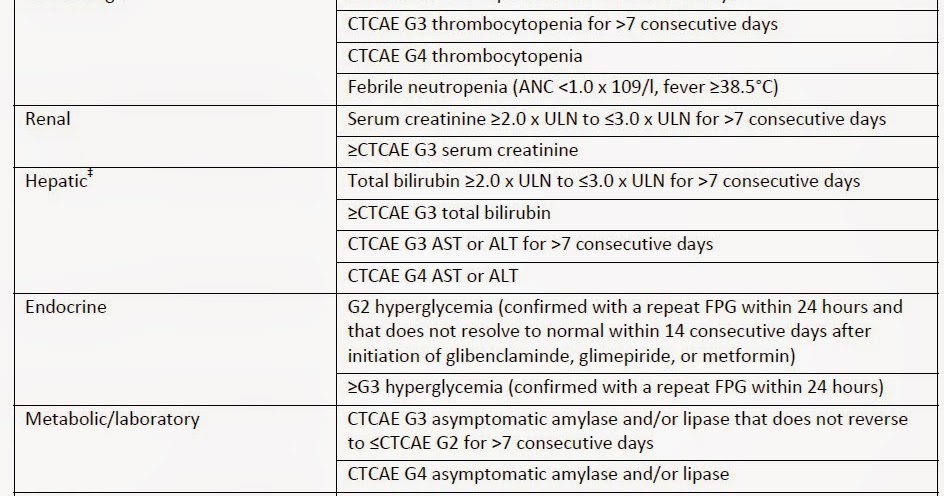
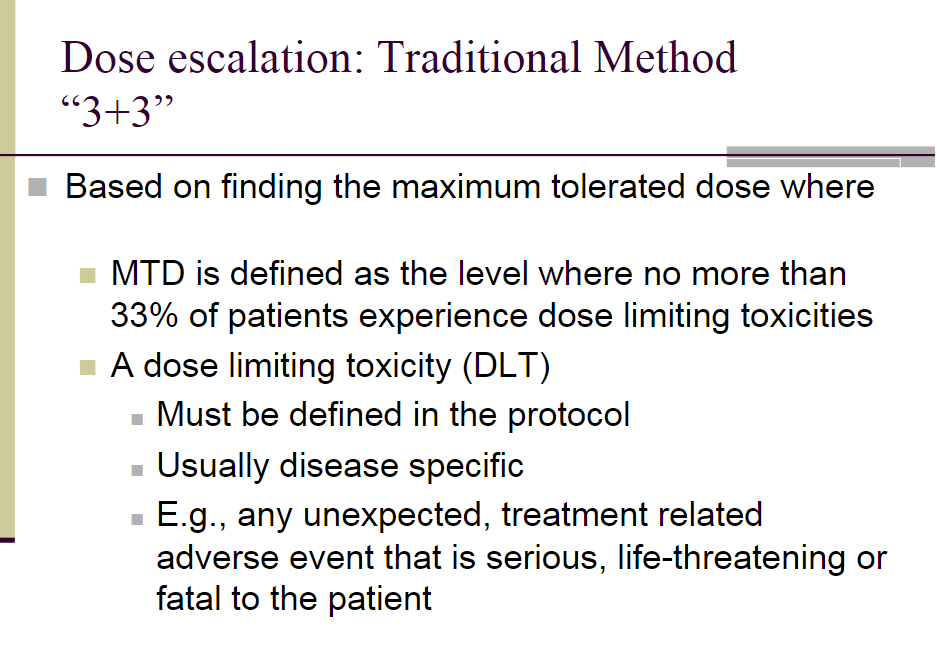
On Biostatistics and Clinical Trials: Dose Limiting Toxicity (DLT) and Common Toxicity Criteria (CTC) / Common Terminology Criteria for Adverse Events (CTCAE)

Safety and tolerability of bosutinib in patients with amyotrophic lateral sclerosis (iDReAM study): A multicentre, open-label, dose-escalation phase 1 trial - eClinicalMedicine

Autolack Dose spritzfertig für Ford DLT Gelb Basislack 1,0 Liter 1000ml : Amazon.de: Auto & Motorrad

Designing phase I oncology dose escalation using dose–exposure–toxicity models as a complementary approach to model‐based dose–toxicity models - Pantoja - 2022 - CPT: Pharmacometrics & Systems Pharmacology - Wiley Online Library

A hybrid design for dose‐finding oncology clinical trials - Liao - 2022 - International Journal of Cancer - Wiley Online Library
Three-plus-three dose escalation design. DLT, dose-limiting toxicity;... | Download Scientific Diagram
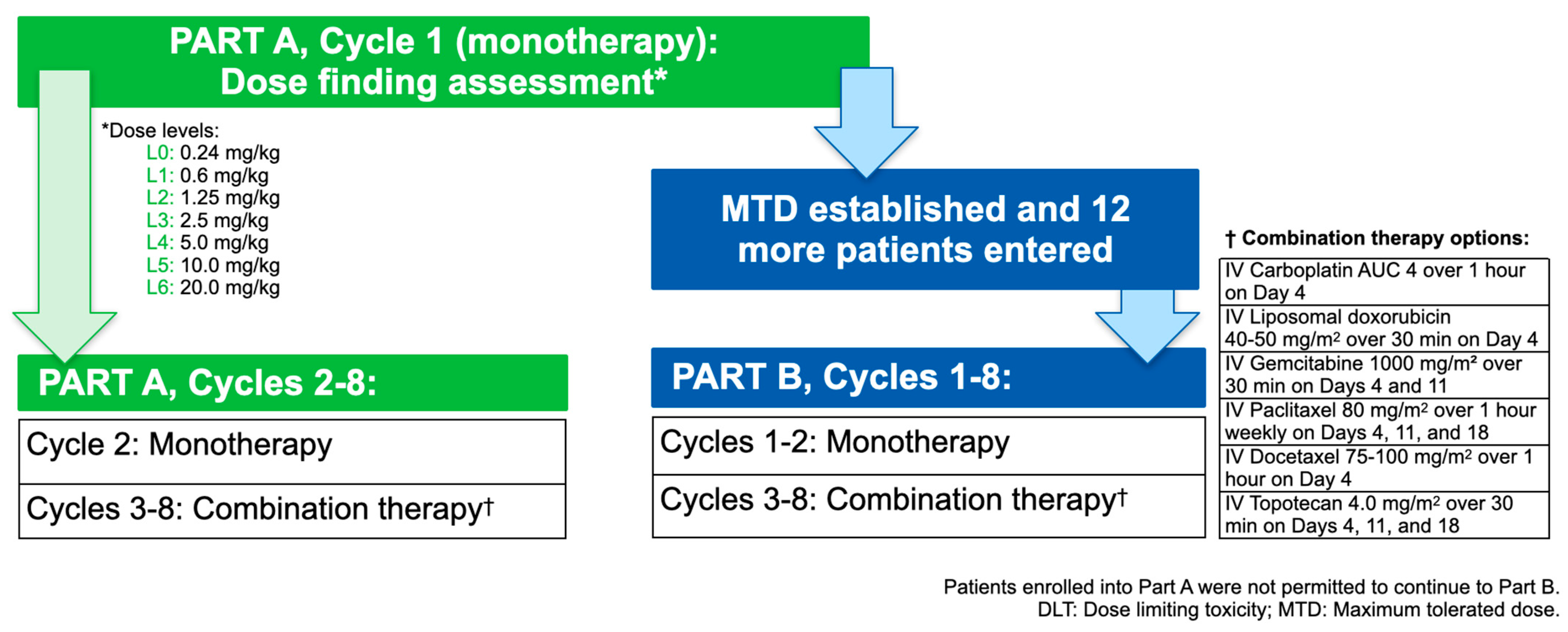
Cancers | Free Full-Text | Maximum Tolerated Dose and Anti-Tumor Activity of Intraperitoneal Cantrixil (TRX-E-002-1) in Patients with Persistent or Recurrent Ovarian Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer: Phase I
A Multicenter Phase I/II Study of Obatoclax Mesylate Administered as a 3- or 24-Hour Infusion in Older Patients with Previously Untreated Acute Myeloid Leukemia | PLOS ONE

Innovative design for a phase 1 trial with intra-patient dose escalation: The Crotoxin study. - Abstract - Europe PMC
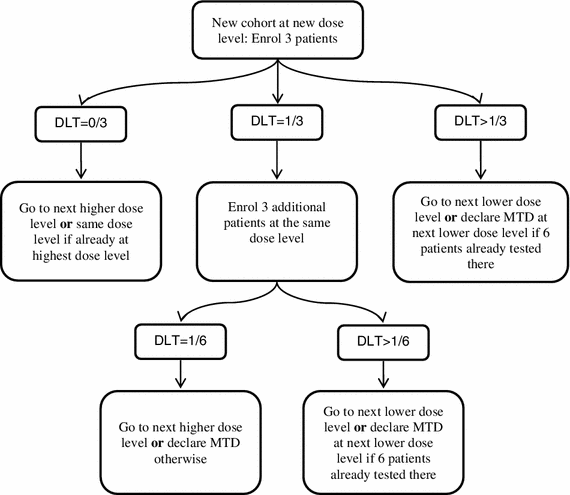
Principles of dose finding studies in cancer: a comparison of trial designs | Cancer Chemotherapy and Pharmacology
![PDF] Dose Finding with Escalation with Overdose Control (EWOC) in Cancer Clinical Trials | Semantic Scholar PDF] Dose Finding with Escalation with Overdose Control (EWOC) in Cancer Clinical Trials | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/07f55deb178b0e088156c61eee7727c9a310acfd/7-Figure4-1.png)
PDF] Dose Finding with Escalation with Overdose Control (EWOC) in Cancer Clinical Trials | Semantic Scholar

Adaptive design for identifying maximum tolerated dose early to accelerate dose-finding trial | BMC Medical Research Methodology | Full Text

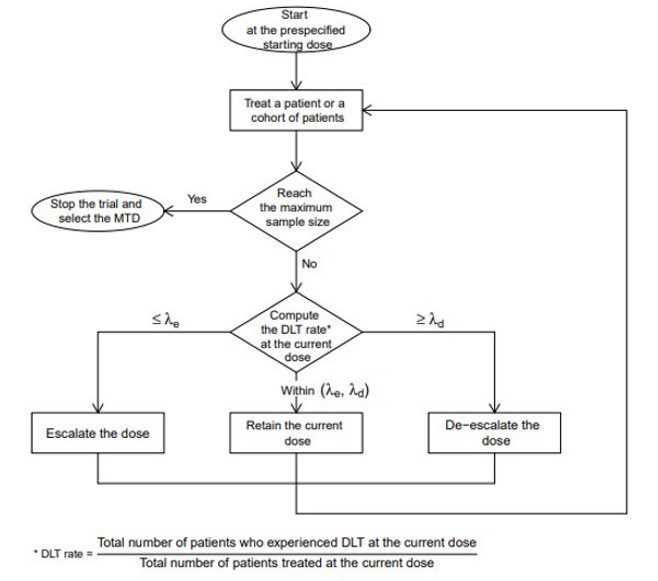
Bayesian Optimal Interval Design: A Simple and Well-Performing Design for Phase I Oncology Trials. - Abstract - Europe PMC

Exposure driven dose escalation design with overdose control: Concept and first real life experience in an oncology phase I trial - ScienceDirect